Introduction
Gastric cancer ranks as the third leading cause of cancer-related mortality globally, characterized by robust metastatic potential. Common metastatic sites include the peritoneum, lymph nodes, liver, lungs, and bone. During metastasis, epithelial-mesenchymal transition (EMT) is a critical step in conferring metastatic phenotypes to tumor cells. The initial phase of EMT involves the disruption of intercellular junctions, such as tight junctions (TJs), adherens junctions, desmosomes, and gap junctions. TJs comprise intrinsic membrane proteins (e.g., claudins [CLDNs], occludin) and cytoplasmic scaffold proteins (e.g., zonula occludens-1 [ZO1], ZO2, ZO3), which collectively anchor cytoskeletal proteins and mediate signal transduction. CLDNs play pivotal roles in maintaining cellular architecture, cell-cell adhesion, and metastasis.
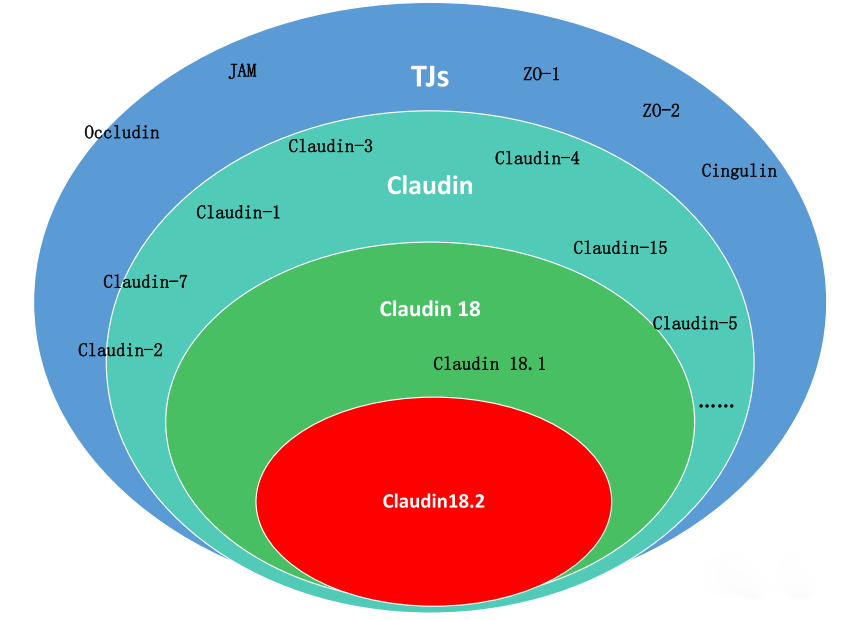
CLDN18.2 is restrictively expressed in terminally differentiated gastric mucosal epithelial cells and on the surface of various tumor cells. In normal gastric tissue, it preserves mucosal barrier integrity by preventing paracellular leakage of gastric acid H⁺. During malignant transformation of gastric epithelium, perturbations in cell polarity lead to surface exposure of CLDN18.2 epitopes and its highly selective, stable overexpression in specific tumors. CLDN18.2 exhibits remarkable cross-species homology (up to 84% in humans, chimpanzees, macaques, mice, rats, dogs, and rabbits), facilitating preclinical safety and efficacy evaluations in drug development.
Molecular Features of CLDN18.2
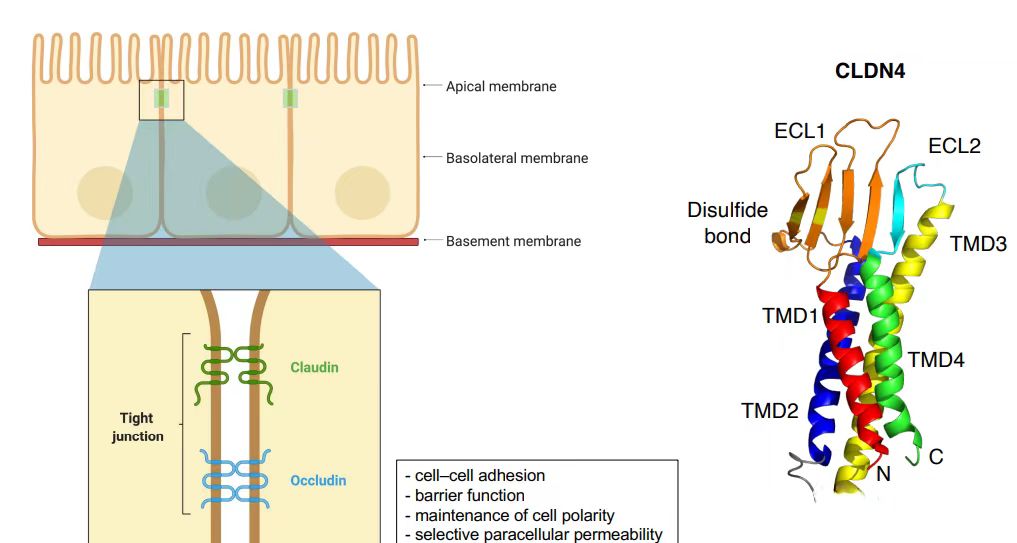
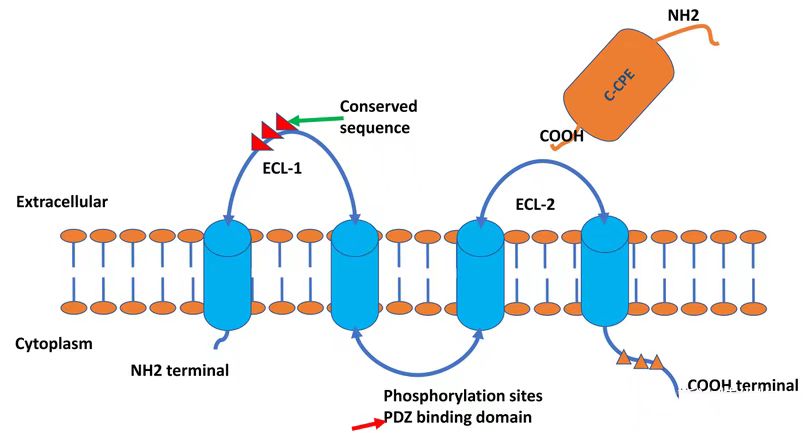
The CLDN18.2 gene is located at human chromosomal locus 3q22, encoding a 261-amino acid tetraspanning transmembrane protein with a molecular weight of 27.7 kDa. Both N- and C-termini reside intracellularly. CLDN18.2 harbors two extracellular loops (ECLs): ECL1 consists of 53 amino acids forming four antiparallel β-sheets, stabilized by a disulfide bond between two highly conserved cysteine residues (β3 and β4). ECL2 comprises 31 amino acids.
Regulation and Expression of CLDN18.2
Phorbol 12-myristate 13-acetate (PMA) upregulates CLDN18.2 expression via protein kinase C (PKC) activation. Pretreatment of gastric cancer cell lines with the PKC inhibitor GF109203X abolishes PMA-induced CLDN18.2 upregulation, confirming PKC dependency. The AP1 transcription factor binding site within the CLDN18.2 promoter mediates PMA-driven expression. Additionally, CpG islands in the CLDN18.2 promoter are fully methylated in non-expressing tissues (e.g., normal lung, peripheral blood mononuclear cells) but hypomethylated in gastric tissues and tumor cell lines (e.g., lung cancer), correlating with its tumor-specific expression.
CLDN18.2-Associated Tumors and Therapeutics
CLDN18.2 was initially identified as a consistently expressed biomarker in gastric cancer and later detected in breast, colorectal, hepatocellular, head and neck, bronchial, non-small cell lung, and esophageal cancers. Immunohistochemical (IHC) analysis of 105 gastric signet-ring cell carcinoma samples revealed 95.2% CLDN18.2 positivity, with moderate-to-strong expression in 64.8% of cases. Notably, 21% of samples exhibited >90% tumor cell positivity.
CLDN18.2 overexpression correlates with thyroid transcription factor (TTF)-negative adenocarcinomas, suggesting its utility as a diagnostic marker for TTF-negative tumors. Beyond primary tumors, CLDN18.2 is highly expressed in metastatic lesions (e.g., 70% in lymph node metastases, 66% in hepatic metastases, 59% in pulmonary metastases) and is implicated in malignant proliferation and chemotaxis. Metastatic lymph nodes display significantly higher CLDN18.2 levels than primary tumors, correlating with poorer prognosis. While the mechanistic link between CLDN18.2 and lymph node metastasis remains unclear, it may involve structural and functional alterations in tumor cell tight junctions.
Therapeutic Advances Targeting CLDN18.2-Positive Tumors
Current strategies include monoclonal antibodies (mAbs), bispecific antibodies, CAR-T therapies, and antibody-drug conjugates (ADCs). As of January 2022, 15 domestic and 28 international clinical trials are underway, focusing on advanced unresectable gastric cancer, metastatic esophageal cancer, pancreatic cancer, and other solid tumors. These include 22 mAbs, 3 bispecific antibodies, 9 CAR-T therapies, and 9 ADCs.
Monoclonal Antibodies
1.Claudiximab: A mouse-human chimeric monoclonal antibody (human IgG1 constant region) developed by Ganymed Pharmaceuticals (Germany), targeting the first extracellular domain (ECD1) of CLDN18.2 with high specificity and affinity. Preclinical studies demonstrate Claudiximab-induced apoptosis in CLDN18.2-positive pancreatic cells, with cytotoxicity proportional to surface expression levels. Synergy with gemcitabine enhances CLDN18.2-mediated antibody-dependent cellular cytotoxicity (ADCC).
2.TST1001: A defucosylated, humanized IgG1 antibody with enhanced FcγRⅢa binding affinity, eliciting stronger ADCC, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP) than Claudiximab. In a Phase I dose-escalation trial, TST1001 monotherapy achieved partial symptom remission within 6 weeks in advanced gastric cancer patients refractory to chemotherapy, PD-1 inhibitors, and VEGF inhibitors. Rapid tumor marker reduction was observed in one patient, with similar efficacy in combination trials.
Bispecific Antibodies
1.AMG-910: A half-life extended HLE BiTE (bispecific T-cell engager) targeting CD3⁺ T cells and CLDN18.2⁺ tumor cells. Primary endpoints include dose-limiting toxicity and adverse events; secondary endpoints encompass pharmacokinetics, objective response rate, duration of response, progression-free survival (6-month and 1-year), and overall survival (1-year and 2-year).
2.Q-1802: A PD-L1/CLDN18.2 bispecific antibody mediating ADCC while blocking PD-1/PD-L1 interaction to activate antitumor immunity. A Phase I trial is ongoing.
Additional Modalities
CAR-T therapies (e.g., CT041, LY011, LCAR-C18S) and ADCs (e.g., CMG901, SYSA-1801, RC118) are under active clinical investigation.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| S0B2072 | Claudin-1 Recombinant Rabbit mAb (SDT-R024) | Host : Rabbit | $45 |
| S0B2070 | S-RMab® Claudin18.2 Recombinant Rabbit mAb (SDT-102-83) | Host : Rabbit | $880 |
| S0B2069 | Claudin18.2 Recombinant Rabbit mAb (SDT-102-24) | Host : Rabbit | $45 |
Source of the article:The Rising Star in Tumor Targets - Claudin18.2 Antibody Now Available